Nano-Emulsion Formulation Overcomes Injectable Clopidogrel Challenges
In our previous blog on nano-emulsion technology, we explained oil-in-water (O/W) and water-in-oil (W/O) types and the benefits each brings in drug formulations. It is because of these benefits – drug solubility, loading and stability, and reduction of injection pain and thrombophlebitis – that drug development teams at pharmaceutical companies are using nano-emulsions to deliver active pharmaceutical ingredient (APIs). To realize cost and time efficiencies on these projects, it’s best to partner with a Contract Development and Manufacturing Organization (CDMO) with proven experience in nano-emulsions.

Emulsol (Left), a proprietary technology, was developed, for production of novel O/W nano-emulsions. It greatly reduces the irritation and injection site pain for a parenterally delivered product. For, this reason, it was used on a project for Clopidogrel IV by 505(b) pathway.
Developing an Injectable Clopidogrel
Co-developed and co-marketed as Plavix® by Bristol-Myers Squibb and Sanofi, clopidogrel was first approved in 1997 and is now one of the leading anti-thrombotic drugs in use. It is indicated for Acute Coronary Syndrome (ACS), as well as myocardial infarction, stroke, and in established peripheral arterial disease. ACS refers to unstable angina, or when the blood supply to the coronary arteries in a patient becomes suddenly fully or partially blocked.
Currently, there is an unmet medical need in the fast onset of P2Y12 agents in patients prior to urgent percutaneous coronary intervention (PCI) procedures. When a patient presents with a suspected coronary event, a 300- to 600-mg loading dose of clopidogrel is frequently administered. The only commercially available dosage forms of clopidogrel, however, are oral tablets in 300-mg and 75-mg strengths. Neither is ideal for administration in an emergency setting.
One drawback in an emergency scenario is that when clopidogrel is delivered orally, it can take as long as 2-5 hours for the medicine to become effective. The several hour delay to reach peak concentration – even though clopidogrel is rapidly absorbed – makes the current tablet delivery method ineffective. Therefore, in an acute, emergency setting a higher dose (300-600 mg), more rapidly acting, injectable clopidogrel dosage form is desirable.
Overcoming Formulation Challenges
Ascendia was selected as the CDMO for this project, in part, because of its experience with poorly soluble drugs. There were multiple challenges associated with developing the injectable formulation:
Clopidogrel’s solubility, physical form, and chemical stability properties. Clopidogrel is a weak base with a pKa of 4.5, and it is practically insoluble in water at neutral pH. The oral tablet composition uses the bisulfate salt form of clopidogrel, which is soluble at gastric pH, but not suitable for injection.
Clopidogrel free base is a semisolid, viscous, oily form. Because of this factor, there are complications related to storage, dispensing, and processing. Plus, the free-base form is chemically unstable and undergoes both hydrolysis and oxidation.
Clopidogrel is a chiral molecule. Only the senantiomer is biologically active. Additionally, chiral conversion to the r-enantiomer can easily occur when clopidogrel is in a liquid dosage form.
There have been development efforts to formulate and clinically test an injectable clopidogrel dosage form. Ligand licensed an injectable clopidogrel formulation to The Medicines Company in 2011.1 Other injectable formulations have been studied clinically.1 Patent literature also discloses attempts to formulate stable parenteral dosage forms.2 Despite the numerous development efforts to date and obvious medical need, an injectable form of clopidogrel has not been successfully developed and approved.
Experienced Team Develops Solution
The experienced team of scientists at Ascendia used the EmulSol technology to develop a novel O/W nano-emulsion formulation of clopidogrel. The ASD-002 nano-emulsion is prepared by a unique high-pressure and high-shear cGMP process in Ascendia’s Class 10,000 (ISO 7) and Class 100 (ISO 5) cleanrooms.
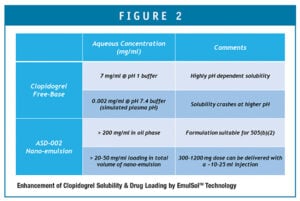
The project had to demonstrate successful formulation of clopidogrel free-base in a nano-emulsion that is suitable for injection, having acceptable chemical and physical stability properties. Because the formulation developed by Ascendia contains no solvent, the risk of injection site pain is significantly less. The clopidogrel drug substance becomes much more soluble (Figure 2) when contained in the oil phase of the nano-emulsion, as well.
To validate the formulation, chemical and physical stability had to be demonstrated. Clopidogrel has several degradation pathways, including oxidation, hydrolysis, and chiral conversion. The degradation pathways of clopidogrel free-base and clopidogrel bisulfate were analyzed in an aqueous solution. Results revealed a means to develop stability indicating analytical methods. The mean particle size of the oil droplet remained a consistent nano-range size after autoclaving and freeze-thaw cycle, which verified the formulation’s physical stability.
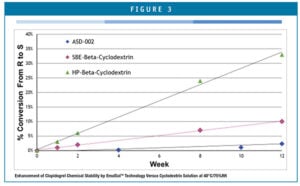 Chiral conversion to the r-enantiomer is the predominant chemical impurity. Accelerated stability studies showed that chiral conversion is kept within United States Pharmacopeia (USP) limits for enough time to provide a commercially acceptable product shelf-life. ASD-002’s stability profile has been compared to other aqueous-based liquid formulations of clopidogrel and demonstrates superior chemical stability with respect to all three major impurities – chiral degradation, oxidation, and hydrolysis, as shown in Figure 3.
Chiral conversion to the r-enantiomer is the predominant chemical impurity. Accelerated stability studies showed that chiral conversion is kept within United States Pharmacopeia (USP) limits for enough time to provide a commercially acceptable product shelf-life. ASD-002’s stability profile has been compared to other aqueous-based liquid formulations of clopidogrel and demonstrates superior chemical stability with respect to all three major impurities – chiral degradation, oxidation, and hydrolysis, as shown in Figure 3.
Conclusion
O/W nano-emulsions are stabilizing drugs, increasing drug loading, reducing injection site reaction, and achieving unique drug PK/PD profiles. Despite unsuccessful attempts in the past by other companies, Ascendia has developed a soluble, stable form of the anti-thrombotic drug clopidogrel in a suitable parenteral form. At the center of the formulation is EmulSol.
The novel O/W nano-emulsion formulation transformed the insoluble and unstable free-base form of clopidogrel to one that has acceptable drug loading and is protected from chemical degradation. The result is that a ready-to-use, nano-emulsion, parenteral form of clopidogrel with a faster and higher PK/PD effect and a higher single dose >300 to 1200 mg is moving forward into development for human BA/BE studies under the NDA 505(b)(2) pathway.
Do you have a poorly soluble drug in your pipeline? Contact us to discuss how we can meet your formulation challenges.
Sources:
1.Ligand Pharmaceuticals press release 03 June 2011.
2.Cushing DJ, Souney PF, Cooper WD, Mosher GL, Adams MP, Machatha S, Zhang B, Kowey PR. Pharmacokinetics and platelet aggregation inhibitory effects of a novel intravenous formulation of clopidogrel in humans. Clin Exp Pharmacol Physiol. 2012;39(1):3-8.
