How Lipid Nanoparticles Overcome Solubility Challenges for Oral and Injectable Formulations
A CDMO with experience in poorly soluble formulations and advanced technologies can help overcome oral and injectable formulation development.
More than 80% of new chemical entities (NCEs) are poorly soluble compounds, making solubilization a critical factor when pharmaceutical and biopharmaceutical companies are formulating and developing drugs. Overcoming poor solubility to efficiently advance the product through the pipeline and into the market on schedule and within budget requires CDMO partners with proven success using lipid nanoparticles (LNPs) as vehicles for drug delivery.
Formulation Technologies
Selecting the optimal formulation technology is the first step on the road to success. Conventional technologies, such as those in figure 1, are proven techniques for numerous commercial drugs in oral tablets, oral liquids, and parenteral formulations. The lack of inherent compatibility with excipients and processing conditions used in manufacturing create obstacles, though. As more hydrophobic or brick dust and/or lipophilic or waxy molecules emerge from the discovery, the situation becomes worse.
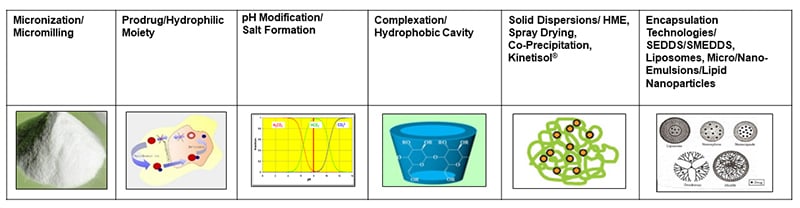 Figure 1:: Technologies for formulating poorly soluble NCEs
Figure 1:: Technologies for formulating poorly soluble NCEs
Formulation Technology Advancements
Polymers have been used in several drug development products. Examples are seen in amorphous solid dispersions (ASD) prepared by hot melt extrusion, spray drying, and co-precipitation.1 Among the benefits of polymers are enhanced stability and solubilization capabilities and ease of handling, and long-term stability.
Lipids are more prevalent because they have improved suitability and greater drug delivery efficiency. Lipids used as proliposomes in dry, free flowing powder, following re-constituted in buffer formed multilamellar vesicles (MLVs) with higher drug loading have shown to improve efficacy in oral formulation.2 Katare have also demonstrated that indomethacin in pro-liposomes improve efficacy when administered orally.3 Lipid-based SEDDS/SMEDDS also have gained significance for improving the efficacy of many marketed drugs.4
Improving NCE Solubility and Bioavailability
Before defining the LNP, drug development teams must understand the Active Pharmaceutical Ingredient (API) nanosizing. For example, nanosizing by microfluidic can create higher energy fine particles in liquid suspension. It appears that nanosizing increases the surface area and in vivo exposure, enhancing the bioavailability of APIs. These are important, as many NCEs possess poor solubility.
Developing them in medium to high dosages may result in unwanted toxicity and side effects, and subsequent long-term health risks. Therefore, finding the appropriate formulation technologies and strategies to help prevent unwanted side effects in clinical studies and in post launch marketed drugs are highly warranted for immediate release, controlled release, and lifecycle management.
Lipid and surfactant-based particulates have been identified as alternative delivery systems to polymeric excipients. Figure 2 clarifies some of the misconceptions and outlines the evolution of these assemblies and their relevance in drug delivery over the years..
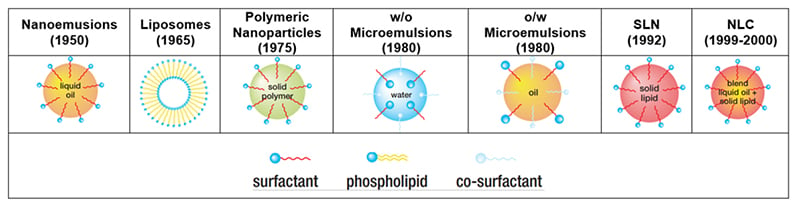
Figure 2: Progression of colloidal drug delivery systems.5
Some clear differences exist between assemblies. For instance, microemulsions and nanoemulsions have different particle sizes, as well as methods for preparations, and/or stability. Macroemulsions and microemulsions differ in particle size, and stability and encapsulation efficiency. Polymeric nanoparticles and lipid aggregates share stability but they are different when it comes to particulates.
Larger lipid droplets such as emulsions and/or macroemulsions (> 1 microns) could lead to Oswald ripening, yielding much larger droplets. Encapsulated drugs can come from the interior core, as a result, ultimately leading to precipitate at the outer particulate surface. For this reason, smaller particle droplets are better suited in drug delivery in which optimal stability, higher drug loading, faster dispersibility, and absorption in GI tract and/or longer systemic circulation are critical.
Solid lipid nanoparticles (SLN) and Nanostructured lipid carriers (NLC)
LNPs are more common in drug delivery than self-emulsifying microemulsions, nanoemulsions, and/or liposomes and have emerged as promising delivery vehicles for a variety of therapeutics. For example, LNPs are used in COVID-19 vaccines to protect the vaccine, carry mRNA and deliver the vaccine to target cells.6 Figure 3 illustrates the similarities/dissimilarities of these lipid assemblies’ drug encapsulation capabilities.
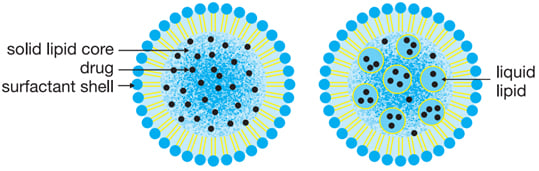 Figure 3: SLN (left) and NLC (right).
Figure 3: SLN (left) and NLC (right).
SLNs and NLCs have higher drug encapsulation efficiency; stability; ease of preparation; biocompatibility; non-immunogenicity; and controlled release, tissue targeting, and maximum drug entrapment efficiency. For these reasons, they are recommended. SLNs primarily created by 0.5-5% surfactants as emulsifying agents, with solid low melting lipids, are spherical in diameter ( > 50 nm). The inner core is saturated fatty acid (solid) embedded with the API. The outer surface is surrounded by emulsifying agents, surfactants or PEG-ylated lipids.
Cold or hot homogenization and solvent injection processes are commonly used to prepare SLNs.7 Ultrasonication or high-speed homogenization, supercritical fluid extraction of emulsions, and spray drying are alternative methods to produce SLNs and NCs.
An NLC is next-generation modified SLN, and are used widely in oral, pulmonary, gene, injectable and topical applications. Structurally, NLCs are composed of both solid (fat) and liquid (oil) lipids at ambient temperature, that help improve drug loading and stability by better accommodating APIs in the interior core comparted to hydrophobic SLN interior core.8, 9
Conclusion
Ascendia Pharmaceuticals has a team of scientists that has developed an effective LNP formulation process. Our formulations to overcome poor solubility utilize three proprietary nanotechnologies.
To learn how Ascendia can help you overcome your drug development challenges, contact us.
References
1. M. Crew, Bioavailability Enhancement – Analysis of the historical use of solubilization technologies. Drug Development & Delivery. 2014, 14, 22-252. V. Nekkani, N. Venkatesan, and G. V. Betageri, Proliposomes for oral delivery: progress and challenges, Curr. Pharm. Biotech., 2015, 16-1-10.
3. O. P. Katare, S. P. Vyas, and V. K. Dixit, Proliposomes of indomethacin for oral administration, J. Microencapsul., 1991, 8, 1-7.
4. L. Wu, Y. Qiao, L. Wang, J. Guo, G. Wang, W. He, L. Yin and J. Zhao, A self emulsifying drug delivery system(SMEDDS) for novel medicative compounds against depression: a preparation and bioavailability study in rats, AAPS PharmSciTech, 2015, 16, 1051-1058.
5. Z. Wen, B. Liu, Z. Zheng, X. You, Y. Pu, and Q. Li, Preparation of liposomes entrapment essential oil from Atractylodes macrocephala Koidz by modified RESS technique, Chem. Eng. Res. Des., 2010, 88, 1102-1107.
6. R. Tenchov, R. Bird, A. E. Curtze, and Q. Zhou, Lipid nanoparticles – from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement, ACS Nano, 2021, 15, 16982−17015.
7. Y. Duan, A. Dhar, C. Patel, Mehul Khimani, S. Neogi, P. Sharma, N. S. Kumar, and R. Vekariya, S brief review on solid lipid nanoparticles: part and parcels of complementary drug delivery systems, RSC Adv. 2020, 10, 26777-26791.
8. N. Nasei, H. Valizadeh, and P. Zakeri-Milani, Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application, Adv. Pharm. Bull., 2015, 5, 305-313.
9. W. Mehnert and K. Mader, Solid lipid nanoparticles: production, characterization, and applications, Adv. Drug Deliv. Rev., 2001, 47, 165-186.
