Amorphous Nanoparticles for Drug Delivery of Poorly Water-Soluble Compounds
Amorphous nanoparticles are a new class of pharmaceutical dosage forms gaining traction in the drug development world. They are increasingly used in pharmaceutical compounds with solubility and bioavailability challenges during the discovery, preclinical, and early clinical development phases. CDMOs with a thorough understanding of amorphous stabilization and nano colloidal properties in relationship to in-vitro and in-vivo performance can help drug development teams advance this dosage form into human clinical testing and commercialization.
What is an Amorphous Nanoparticle?
Amorphous nanoparticles possessing the advantages of nanoparticles and amorphous materials in enhancing solubility and bioavailability of insoluble drugs. Advantages include:
♦ Improving solubility and bioavailability
♦ Suitable for injectable dosage forms
♦ Eliminating food effect
♦ Increasing drug loading
♦ Eliminating co-solvents
♦ Dose reduction
♦ Better dose flexibility and accuracy
♦ Easy swallowing for pediatric or geriatric populations
Nanosizing enhances drug release rate by increasing the dissolution surface area. Amorphous formation can significantly increase the solubility of poorly soluble drugs in an aqueous medium. Saturated solubility and dissolution rate of amorphous nanoparticles can potentially be increased by more than thousand-folds relative to its crystalline counterpart. Because of this, the GI absorption and bioavailability of orally administered drugs can be dramatically improved. This is particularly true for poorly water-soluble drugs classified as BCS IIa (dissolution limited absorption) and BCS IIb (solubility limited absorption).
Amorphous Nanoparticle Dosage Forms
The intrinsic solid state metastability of amorphous materials and colloid nature of nanosuspension dosage forms warrant a rational design of amorphous nanoparticles dosage forms. It is necessary to extend the application from discovery to clinical testing and finally to commercial application.
Defining the target product profile requires gathering data from multiple sources. Examples include a strong understanding of compound properties, solvent solubility, miscibility/compatibility with carrier/stabilizer, therapeutic dose in relevant to drug loading, route of administration, and patient population. A stable dosage form with enhanced drug solubility and bioavailability can be designed with this knowledge.
A viable candidate for amorphous nanosuspension has the characteristics of BCS Class II and IV compounds. These traits include low solubility, amorphous form with a high Tg, a requirement for injectable with high drug loading/solubility ratio, oral dosage form with low bioavailability, and a strong tendency of food effects. A decision guide to determine the suitable form of the nanosuspension and selection of process technology are outlined in figure 1.
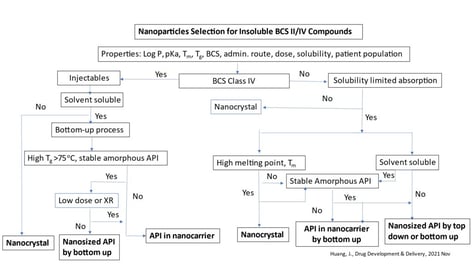
Figure 1: Decision guide.
Selecting the Best Formulation Principle
Based on solid state properties of the API, there are two amorphous nanoparticles routes:
1) Nanosized amorphous drug surrounded by stabilizer(s)
2) API dispersed in a nanosized carrier at a molecular level.
Amorphous API can be nanosized by a traditional top-down milling process if it meets two criteria. First, it must be reproducibly manufactured. Second, it must possess characteristics that can maintain its physical-chemical properties during process, storage, and in-vivo physiological conditions. Ascendia Pharmaceuticals is using nanoparticles, such as high-pressure homogenization, micro-fluidization, sonication, and wet milling methods, to solve customers’ challenges.
If an API forms a fragile glass with a Tg of <75°C, and readily recrystallizes during storage or in-vivo dissolution, excipients can be used to form a multiple-component amorphous system. The excipients help stabilize and inhibit the amorphous drug from crystallization at its solid or aqueous states. An amorphous nanoparticle with a carrier, such as polymer, lipid, or surfactant, is necessary to disperse API at a molecular level in the matrix to stabilize the amorphous API.
A bottom-up process can facilitate drug dispersion in the carrier and formation of amorphous nanoparticles, when the API is molecularly dispersed. Ascendia Pharmaceuticals is using precipitation, co-precipitation, or nano-emulsification methods in its solvent removal processes when using the bottom-up technology.
Formulation and Process Development
Formulation and process development for nanosized amorphous drug nanoparticles prepared by the top-down process are similar to crystalline nanosuspension. A formulation screening study is conducted under a miniature scale to find the suitable stabilizer(s). Different surface properties of API, such as surface charge, hydrophobicity, functional groups responsible for ionic, hydrogen bond, and Van der Waal interactions, may demand different stabilizer types and levels. The performance of nanosuspensions should be confirmed by in-vitro studies.
Initial screening studies to select carrier(s) and stabilizer(s) for amorphous nanoparticles where a drug is dispersed in a nanosized carrier at a molecular level are similar to those for amorphous solid dispersions. There are three primary goals of a screening study:
♦ Determine a polymer and/or surfactant is physically miscible and chemical compatible with the drug
♦ Can load a reasonable amount of drug in nanoparticle matrix
♦ Enhance solubility and stability of insoluble compound in-vitro and in-vivo
Matrix-type amorphous nanoparticles can be prepared using a bottom-up process, high-shear mixing, high-pressure homogenization, or combination of a bottom-up process with a bottom-down nanosizing process. The drug is dissolved in an organic solvent(s) with other stabilizer excipients, which are induced to precipitate by introduction of non-solvent(s). Variation of formulation and process parameters can generate amorphous drug nanoparticles of different particle size that can be further incorporated into dosage forms by a downstream process.
Developing an Amorphous Nanoparticle Process
Experienced CDMOs will establish a process (figure 2) to efficiently develop an amorphous nanoparticle. Ascendia Pharma has used five approaches, depending upon the project.
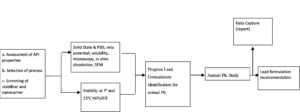
Figure 2: High-level flow chart for amorphous nanoparticles prototype formulation development.
Antisolvent Precipitation / Co-Precipitation Method – A common bottom-up technique, this method dissolves the API and stabilizer(s) in a common solvent, induces nanoprecipitation by mixing the solvent solution containing API/polymer with an antisolvent, and removes the solvent by evaporation or ultrafiltration. Optionally, nanosizing is done using a high shear/pressure/ultrasonic/extrusion process.
Nano-Emulsification Method – This approach disperses one liquid phase into another immiscible liquid phase to generates a uniform mixture of nanodroplets. These two immiscible liquids typically consist of an organic (oil/solvent) phase and an aqueous (water) phase in the presence of a stabilizer, such as surfactant, co-surfactant, and polymer (W/O emulsification).
Wet Milling Technology – A top-down process, wet milling is commonly used for nanocrystal suspension preparation. It can be used to wet mill amorphous APIs into nanoparticles, if the amorphous drug has enough stability during the process and storage. A combination of wet milling with a bottom-up antisolvent precipitation process is an alternative.
High Pressure/High Shear Technology – Similar to wet milling technology, high pressure homogenization, also known as micro fluidization, can achieve nanosuspensions with narrow particle size distribution as a top-down process. Alternatively, a combination of bottom-up and top-down process can be utilized.
Ultrasonication – Ultrasonication (>20 kHz) applies sound energy to agitate particles in a liquid to improve mixing and mass transfer locally at the contact surface. It can be conducted using an ultrasonic bath or an ultrasonic probe.
To reduce suspension particle size, ultrasonication can be used in the top-down process. It is also part of a bottom-up method to control particle size and crystallinity at time of solvent/nonsolvent mixing for preparation of nanoparticles. The ultrasonic process may generate crystalline or amorphous nanoparticles with varied particle size distribution.
Ascendia Success Story: PLGA Nanoparticles for IV Infusion
A drug development team approached Ascendia Pharma with a compound classified as BCS IV (low solubility, low permeability). The compound was targeted for IV infusion due to low oral bioavailability. Because it has a narrow therapeutics index, a sustained-release nanoparticle formulation was the goal. It would enable IV infusion, reduce Cmax, and increase half-life to reduce compound toxicity and increase drug exposure at the target organ.
Based on the assessment of the compound properties, Ascendia’s scientific team selected Nanosol® for screening and in-vitro assessment of the nanoparticle sustained-release formulation. An O/W nano-emulsification was explored using biodegradable PLGA polymers as the nanocarrier for the compound. Several nanosuspension prototype formulations with in-vitro release rate ranging from <1 day to >2 weeks were developed and tested for stability and animal PK. The lead nanoparticle particle size was determined to be ~100-200 nm (Figure 3).
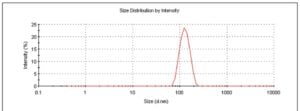
Figure 3: Particle size of the lead nanoparticles in compound developed by
Ascendia Pharma was ~100-200 nm.
The lead prototype nanosuspensions developed achieved a corresponding in-vivo long half-life up to one day to two weeks. There was a decrease in drug toxicity while drug exposure increased at the target organ site in animal models.
Contact us to discuss how our team of specialists can help with your drug development pipeline.
