Sterile Injectable Drugs Defined
Injectable drugs must be sterile, as they bypass the body’s natural defenses against pathogens. This means that the sterile injectable manufacturing process is subject to stringent regulation, and facilities that manufacture these drugs must be specially equipped for aseptic production. Below, we cover the definition of sterile injectables, as well as their manufacturing process and the current issues facing the US injectable market.
What Is a Sterile Injectable Drug?
Sterile injectable drugs are a type of parenteral drug, which is a class of drugs that are not taken orally and absorbed through the digestive tract. These drugs are more challenging to administer, but the advantage is that they can be absorbed quickly and they eliminate the risk of first-pass effect; 100 percent of the drug is bioavailable.
Sterile injectable drugs all involve injections, but there are a number of different types of injections used for administering these drugs. They include:
Subcutaneous injections – Insulin and live vaccines are injected subcutaneously. Such injections require only a short, thin needle, as they are administered into the fat tissue between the skin and muscle.
Intramuscular injections – Intramuscular injections are administered deep into a muscle (often the deltoid, vastus lateralis, or ventrogluteal muscles) so they can be absorbed by the blood vessels. Annual flu shots are an example of an intramuscular injection.
Intravenous injections – Also known as IV injections, these sterile injectables are delivered directly into the bloodstream via injection into a vein.
Intraperitoneal injections – These injections are administered into the peritoneum or body cavity. Certain types of chemotherapy are delivered via intraperitoneal injection.
Intraosseous injections – Intraosseous injections are rarely used in medicine and are typically only considered when IV access is not possible. These injections involve inserting a needle into the bone marrow of a large bone.
Intracardiac injections – This is another type of injection that is typically only used in emergency situations; it involves injecting a drug directly into the heart.
Intraarticular injections – More commonly known as a joint injection, an intraarticular injection administers a drug into a joint. Often, this is used in the treatment of inflammatory joint conditions like rheumatoid arthritis, carpal tunnel syndrome, and gout.
Intracavernous injections – Intracavernous injections are administered at the base of the penis, typically to treat erectile dysfunction.
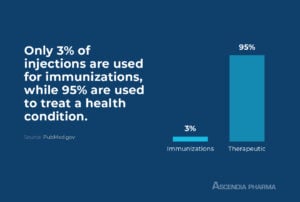
Only three percent of injections are used for immunizations, while 95 percent are therapeutic (used to treat a health condition); an increasing share of the sterile injectable drug market belongs to biologics, although there are also many small molecule drugs that are administered via injection.
Nearly all injectable drugs are sterile; the exception to this is veterinary drugs used for euthanasia. Thus, when we talk about injectables, the fact that they are sterile is a given. Sterile means there are no living microorganisms in the end-product. Drugs that are administered orally should also be manufactured and handled carefully to avoid contamination, but if they did contain bacteria, the digestive system would have a good chance of killing the pathogen before it causes illness. This is not the case with injectable drugs, which is why they must be sterile.
Sterile Injectable Manufacturing Process
One of the risks of injectable drugs is infection. This could occur because proper aseptic practices were not followed in administering an injectable drug (I.e., a contaminated needle was used or the injection site was not disinfected before the needle was inserted, allowing pathogens to enter the bloodstream), or because the drug itself was contaminated. In modern medicine, the latter is exceedingly rare because the sterile injectable manufacturing process is so highly regulated, but it does occur on occasion. The product may be contaminated during manufacturing or filling, or the packaging could become compromised after sterile injectables are shipped.
Injectable drugs can be made sterile in one of two ways: through an end-to-end aseptic manufacturing process or through terminal sterilization.
1. Terminal Sterilization for Sterile Injectable Drugs
Terminal sterilization focuses on producing a sterile end-product. Although the entire manufacturing process still follows strict standards to reduce the risk of contamination, the actual sterilization occurs at the end of the manufacturing process, so these standards aren’t as stringent as those required for aseptic manufacturing.
Terminal sterilization often uses a steam autoclave to expose a drug to heat, killing any pathogens. Gamma-ray radiation may be used as an alternative to steam. These methods are used because they sterilize both the outside container and its contents. Since containers are sealed before terminal sterilization, there is no way to introduce pathogens after sterilization unless the medication is tampered with or the packaging is compromised. There are also some sterilization methods that are used to kill microorganisms on surfaces, like ethylene oxide gas, which can sterilize pre-filled syringes.
Injectable drugs that are manufactured using terminal sterilization cost less to produce; because the process is less specialized, there are more manufacturers that produce these types of injectables than manufacturers that have the ability to produce injectables with an aseptic process.

2. Aseptic Sterile Injectable Manufacturing
Many sterile injectables, particularly biologics, must be manufactured using aseptic protocols. This is because they are not stable enough to withstand terminal sterilization. Aseptic manufacturing is a carefully controlled process that strives to eliminate the potential for contamination at every step of manufacturing. This is critical because, unlike with terminal sterilization, there is no final step in which these drugs are sterilized in their containers before being shipped.
Aseptic sterile injectable manufacturing requires:
- – Sterile raw materials
- – Sterile formulation
- – Sterile containers and closures
- – Sterile filling
- – Sterile packaging
Ideally, all of these add up to a sterile end-product, even without terminal sterilization.
While the drugs themselves cannot be sterilized, their containers and all of the equipment used to manufacture these drugs can be, often using the same methods used for terminal sterilization. The aseptic manufacturing process for sterile injectables takes place in a cleanroom, with personnel who wear full-coverage protective clothing to eliminate the potential for shedding pathogens in the vicinity of sterile injectables as they are being manufactured.
Although aseptic manufacturing does not sterilize drugs, it does filter them to remove microorganisms. Testing is performed at different points in the process to ensure an end-product that is free of pyrogens (pathogens that cause fevers). If, after filtering, a batch still has microorganisms present, it is discarded.

Sterile Injectable Drugs Market
By all measures, the sterile injectable drugs market is growing leaps and bounds, with the market expected to reach a valuation of $901.3 billion by 2025, with a CAGR of 11.1 percent.
An aging population, combined with the expanding market for biologics, are driving much of this growth, as is a population that is experiencing a longer lifespan without necessarily a longer healthspan—in other words, people are living longer, but they are often living with a number of chronic illnesses. Another factor is government initiatives for the development of new drugs, and the faster approval process for sterile injectables versus other types of drugs.
This increased demand in the US injectable market, along with quality issues in the manufacturing process, has led to frequent sterile injectable drug shortages, particularly for generics. Such drug shortages can be dangerous and have a significant impact on patient care. In 2018, the FDA implemented the New Inspection Protocol Project (NIPP) in hopes that closer oversight would eliminate many of the quality issues that halt production by causing facilities to lose licenses, operate under restricted manufacturing orders, or even close their doors permanently. Identifying concerns earlier and taking a proactive—rather than reactive—approach can prevent many drug shortages.
Beyond the United States, the worldwide market for injectables is facing similar challenges—there is an increased demand for sterile injectables, but there aren’t enough manufacturers to meet these demands because of the complexity of the manufacturing process and governmental regulations required to ensure patient safety. Injectables are frequently recalled, causing shortages of critical medications. In Australia, a plan for mandatory drug shortage reporting was rolled out by the Therapeutic Goods Administration (TGA) to address these concerns; the EU monitors such shortages through the European Medicines Agency (EMA).
Pharmaceutical Injectables at Ascendia Pharma
At Ascendia Pharma, we specialize in parenteral dosage forms, including sterile injectable drugs. We manufacture cGMP clinical batches of sterile injectables for clinical trials, which means meeting stringent FDA guidelines for quality and safety. Phase 1 manufacturing demands strict sterility and non-pyrogenicity to prevent severe harm or life-threatening health risks to patients participating in clinical studies. Our team understands these demands and has the experience and equipment to ensure that end-products are unadulterated and free of all pathogens.
Our manufacturing facility has both Class 100 (ISO 5) and Class 10,000 (ISO 7) cleanrooms, as well as advanced isolator systems that minimize the risk of contamination from personnel and separate the cleanroom from the aseptic processing line. We are equipped to provide terminal sterilization by steam autoclave for stable sterile injectable drugs, as well as aseptic manufacturing for biologics and other sterile injectables that are not suitable for terminal sterilization.
Learn More About the Sterile Injectable Manufacturing Process
Contact us today to discuss your sterile injectable drug formulation and manufacturing needs.


