Oral Controlled Delivery of Poorly Water-Soluble Drugs
Only one in 5,000 discovery compounds will reach the market. In the most recent Formulation Forum, Ascendia Pharma CEO Jim Huang, PhD explains that a key reason for that poor success rate is a significant increase in the percentage of new chemical entities (NCEs) with poor physical, chemical, and biopharmaceutical properties (BCS II and IV) in the drug pipeline. He also outlines how to increase those percentages.
As explained in the article published by Drug Development & Delivery, about half of drugs on the market and nearly 90% of molecules in the discovery pipeline are poorly water soluble. Poor solubility can lead to low bioavailability, resulting in suboptimal drug delivery, ineffective drug efficacy,
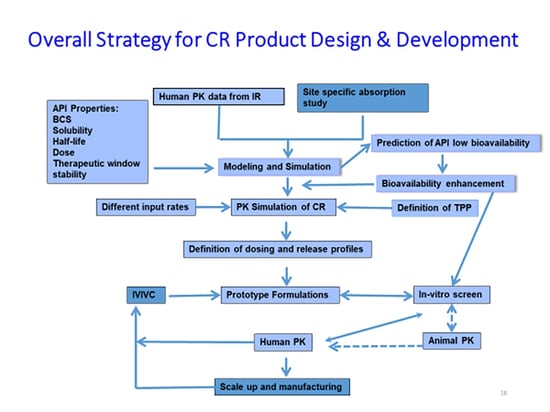
and side effects. As a result, various drug delivery nanotechnologies, such as nano-suspensions, lipid microemulsions, nano-emulsions, and amorphous solid dispersions, have proven critical in overcoming these bioavailability challenges.
The column also details how controlled-release formulations improve the safety and efficacy of NCEs that otherwise might fail due to safety and efficacy reasons. Read the entire Formulation Forum on the Drug Development & Delivery website.
