How to Develop an Effective Formulation Strategy for Early Stage Drug Candidates
Selecting lead compounds during the discovery process helps to best decide on drug candidates – primary and backup – for preclinical development. To help choose the most appropriate candidate and pathway, in vivo studies take on great importance and require a drug development team familiar with this early stage. The question becomes, “What is the best route – in-house or an experienced contract development and manufacturing organization (CDMO)?”
Many large pharma corporations have established their own teams of scientists to conduct formulation research for early stage compounds. Building this drug development team in-house can require a significant financial commitment. Academia, biotech companies, and start-ups in the drug discovery market have also invested in scientists to address the challenge but they, too, have efficiency questions.
There are CDMOs who specialize in pharmacology studies that offer formulation services. Many, though, use standardized processes that result in poorly characterized formulations and may include excipients, such as N,N-Dimethylacetamide (DMA) and N-Methyl-2-pyrrolidone (NMP), that might not be acceptable for in vivo use in some animal models. Because of this, the best solution is to partner with a CDMO with proprietary technologies, a state-of-the-art facility, and understands the challenges associated with the discovery stage.
‘Universal Formulation’ Identification
The holy grail of discovery formulation is identifying a “universal formulation” for a structurally similar lead series. The reason is that it simplifies differentiating in vivo behavior between a number of leads. To accomplish this approved process, scientists must recognize and solve the intrinsic limitations of discovery-stage formulations, such as limited quantities of drug candidates. They must also accept the reality that discovery research brings with it risks such as:
Drug substance purity – Optimizing drug substances is critical yet difficult during the early stage. Drug substance purity is primarily determined by the active pharmaceutical ingredient (API) or the base drug without excipients. Understanding the importance of purity during the discovery stage can optimize the drug’s therapeutic effect inside the body.
Key physicochemical properties of compounds – In early stage development, interaction of compounds, such as log P or pKa, is not usually determined experimentally. This is relevant because the physical environment determines physicochemical properties and the interaction is determined by the interplay of the structural properties. For example, pKa determines the degree of ionization, and it has a major effect on solubility and permeability. Additionally, drugs with a log P value higher than its lipophilic are better absorbed by cells in the body.
Unknown solid state properties – Crystallinity, polymorphism, and other solid state properties all behave differently. Therefore, it is critical for the success of a drug that scientists screen for the various properties to characterize them properly and determine the best solid form.
Availability – The limited accessibility of a drug substance(s) during early stage development limits the number and precision of solubility measurements that can be performed.
Drug substance stability – The drug substance in formulations must be fully evaluated during the early stage. If not, the scientific team will not have a complete picture of how the product will perform.
High-performance liquid chromatography (HPLC) assays – The rapid development of non-validated gradient methods and other HPLCs create a degree of inaccuracy in solubility measurements. Therefore, scientists may not have a reliable indication of drug stability during the discovery stage if the assays are not accounted for.
Experience in the Field
Because of these factors, it is vital that CDMOs that support discovery and preclinical research have the requisite pre-formulation, formulation, and biopharmaceutics expertise. They must also have the proprietary methodologies to develop formulations from small quantities of drug candidates for different routes of administration, whether it is oral (PO), intravenous (IV), inhalation (IN), intramuscular (IM), or subcutaneous (SC).
One effective pathway to drug development involves completely understanding the biopharmaceutical and physical-chemical properties related to drug dissolution, absorption, and the disposition process in the body while taking advantage of advanced formulation technologies. A rational formulation design can be explored with guidance from a decision tree, such as the one Ascendia has developed and is shown in figure 1.
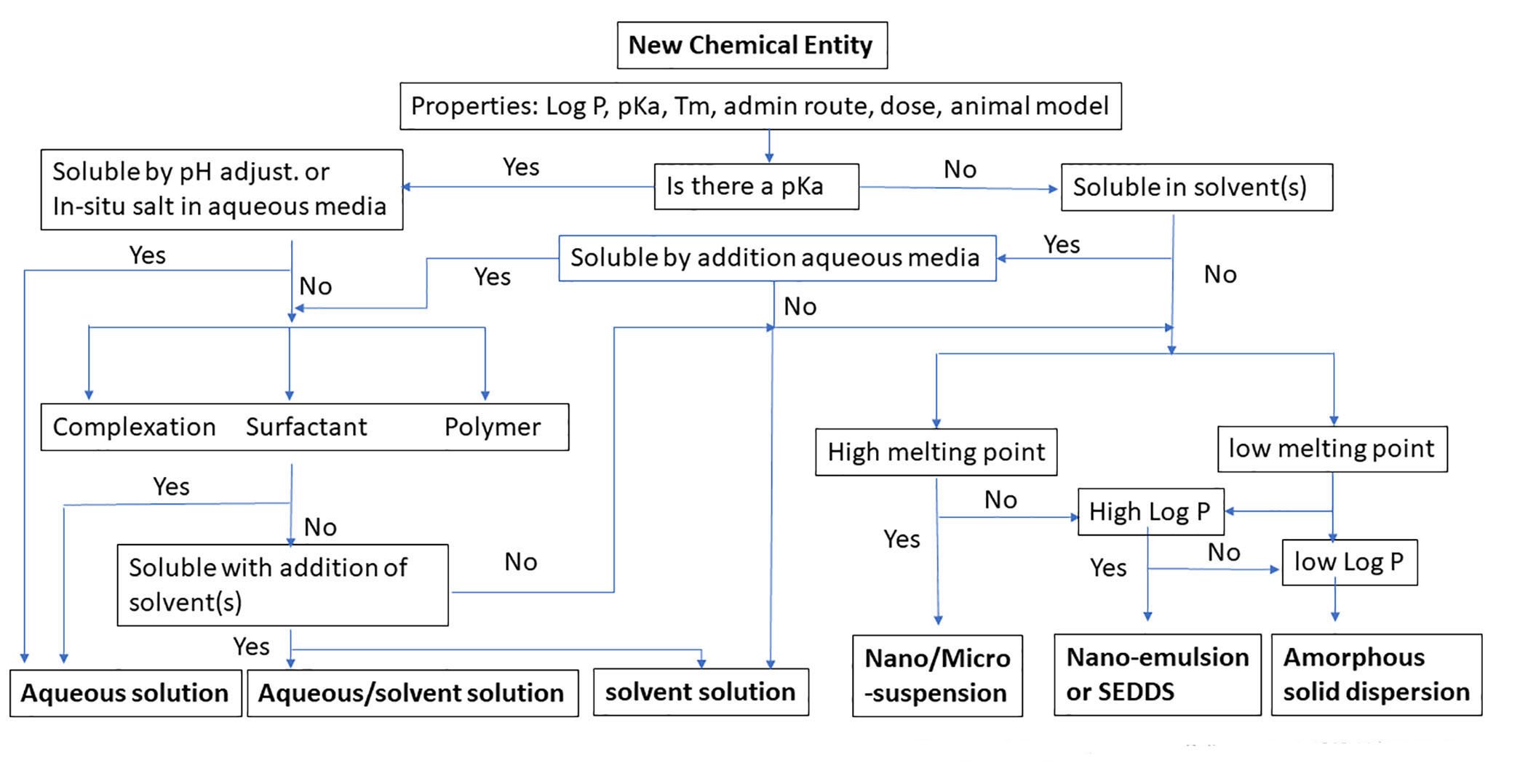 Figure 1
Figure 1
Your Optimal CDMO Partner
CDMOs that recognize and overcome the intrinsic limitations of discovery-stage formulations and the risks associated with discovery research will prove to be the best partners for pharma and biotech companies during the discovery stage of new drug candidates. They will help ensure the key outcomes from early stage formulation studies can support the emerging target product profile.
Another benefit is that valuable intellectual property may be developed when non-obvious biopharmaceutical effects are discovered during formulation development. The result is an enhanced new drug entities’ patent portfolio that extends beyond the basic composition of matter filings.
Are you looking for a partner to help develop a research strategy for your discovery-stage new drug candidates? Contact the Ascendia Pharma business development team at 732.640.0058 to discuss how we can help.
