How Nano-formulations Aid in Advancing Insoluble Drug Candidates
 Pharmaceutical and biotech companies must overcome myriad of obstacles as they travel down the drug development pipeline. Certainly, the statistics are not in their favor. Only one in 5,000 discovery compounds will reach the market, and one in every five drug candidates will gain approval.1
Pharmaceutical and biotech companies must overcome myriad of obstacles as they travel down the drug development pipeline. Certainly, the statistics are not in their favor. Only one in 5,000 discovery compounds will reach the market, and one in every five drug candidates will gain approval.1
Another dilemma is the time to market for drugs. On average, industry professionals estimate that it takes about 14 years from drug discovery to product launch. For these reasons, it isn’t surprising that it costs in excess of $1 billion for a new chemical entity (NCE)2 to be developed and for a new medicine to be introduced to the market.
Such statistics certainly prove to be daunting for any pharmaceutical or biotech company. They are particularly concerning to emerging companies. To alleviate those issues, many start-ups in early-stage drug development rely on contract development and manufacturing organizations (CDMOs), including Ascendia Pharmaceuticals.
Not all CDMOs are alike, however, as some are more experienced in certain aspects than others. After all, drug development is a complicated process that involves collaboration, technical experience, and years of working knowledge for it to end with success.
Our focus is to provide formulation and cost advantages to reduce the preclinical phase time. A dramatic rise in the number of NCEs with inadequate physical, chemical, and biopharmaceutical properties (BCS II and IV) in the drug pipeline has played a major part in the aforementioned failure rates in development.3
Poorly Water-soluble Compounds
Nearly half of the drugs on the market currently, and almost 90% of molecules in the discovery pipeline, are poorly water soluble.4 Developing a stable solution formulation with reasonable drug loading for poorly water-soluble compounds with chemical instability is no easy project. Our specialized team of scientists have experience in this area and can recognize the most effective approach. Often, incorporating a nano-formulation strategy may resolve the drug-loading and bioavailability issues for such compounds, while adding minimum complexity to the manufacturing process, saving time and money.
That knowledge allows us to understand that poor solubility is often the primary reason associated with low bioavailability. In this scenario, there is the real possibility of suboptimal drug delivery, ineffective drug efficacy, and side effects. To help reduce this possibility, nano-suspensions, lipid microemulsions, nano-emulsions, amorphous solid dispersions and other drug delivery nano-technologies can be used.
Effective Formulations = Better Assessments
Using some of these drug development platforms, we have seen effective formulations with good bioavailability to enable better assessment of the pharmacology, toxicology, safety, and efficacy properties of a compound during drug discovery and development. Results will be evident via the positive pharmacokinetics, pharmacodynamics, and toxicological profiles of the drug candidate assessed, with respect to the biological response to specific drug targets.
Poor water-solubility is a common characteristic of drug candidates in pharmaceutical development pipelines today. Various processes have been developed to increase the solubility, dissolution rate and bioavailability of these active ingredients belonging to BCS II and IV classifications. Within the past 10 years, nano-crystal delivery forms and amorphous solid dispersions have become well established throughout the pharmaceutical market.
Customized Nano-formulations
Depending on a compound’s physical, chemical, and biopharmaceutical properties, a rational formulation design can be explored with guidance from a decision tree. Numerous drugs associated with poor solubility and low bioavailability have been successfully formulated into drug products for clinical studies by a suite of available formulation technologies (Figure 1).
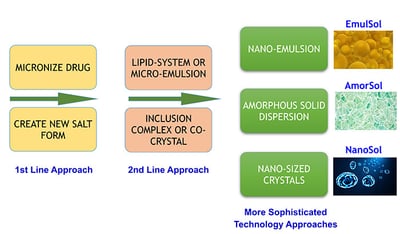
Figure 1 Ascendia Pharmaceuticals specializes in the application of nano-technology approaches to develop unique pharmaceutical products. Our state-of-the-art technologies for formulating poorly water soluble compounds provide sophisticated formulation options for our clients, including Emulsol and Nanosol.
Emulsol
Emulsol, a technology for production of oil-in-water nano-emulsions, is developed using a high-shear homogenization or a micro-fluidics process. Ascendia leverages an innovative nano-emulsion process that does not use organic co-solvents, and only a small amount of surfactants. Taking this approach means Ascendia nano-emulsions are much more suitable for pharmaceutical applications.
One example where a nano-emulsion is beneficial is to deliver a poorly palatable drug in liquid form for a pediatric development program. A nano-emulsion can also greatly reduce the irritation and injection site pain for a parenterally delivered product. It can be used to develop a topical formulation with superior clarity and bioavailability properties, as well. In addition, nano-emulsions can be dehydrated (i.e., lyophilized) and incorporated into solid oral dosage forms.
Nanosol
Another technology for the production of nano-sized drug particles is Nanosol. Formulation of a drug substance in nano-particle form significantly increases the surface area for dissolution. A ten-to-twenty-fold increase in surface area can be realized by reducing particle size from a micronized drug substance to a nanonized drug substance.
Ascendia uses a bead-milling or high-shear homogenization process for production of nano-particles and can investigate the impact of nanonization with small amounts of drug substance. This nano-particle technology can enable pharmaceutical products with enhanced drug loading, bioavailability, reduced food-effect and more rapid onset-of-action.
To learn more and speak to a member of the Ascendia Pharmaceutical business development team, simply complete the form or contact us directly at 732.640.0058.
REFERENCES
- PhRMA July 2013 Profile.
- Dickson M, Gagnon JP. Key factors in the rising cost of new drug discovery and development. Nat Rev Drug Disc. 2004;3: 417-429.
- Li Di, Kerns E. Profiling drug-like properties in discovery research. Curr Opinion Chem Biol. 2003;7:402-408.
- PharmaCircle: Water Insoluble Drug Development: Strategies, Technologies, Case Studies, March 2011.
