EmulSol®
Nano-Emulsion Technology
EmulSol is a technology for production of oil-in-water nano-emulsions. Nano-emulsions have droplet sizes in the range of 50 to 500 nanometers, and are stable for the intended shelf-life of the product. Ascendia® produces nano-emulsions using a high-shear homogenization process. After a suitable oil phase is chosen (based on chemical compatibility and solubility with the drug substance), a mixture of the oil, water, and the drug substance is processed through the homogenizer creating a fine suspension of the oil droplets in a water phase. As the drug substance is significantly more soluble in the oil phase, than in the water phase, the vast majority of the drug is solubilized within the interior of the oil droplets. Thus when the nano-emulsion is delivered to the body, the drug substance is more readily bioavailable.
Ascendia’s nano-emulsion process is novel in that it uses no organic co-solvents, and a minimal amount of surfactant excipients. By minimizing surfactants, and eliminating solvents, Ascendia’s nano-emulsions are suitable for pharmaceutical applications. A nano-emulsion can greatly reduce the irritation and injection site pain for a parenterally delivered product. A nano-emulsion can be used to develop a topical formulation with superior clarity and bioavailability properties. In addition, nano-emulsions can be dehydrated and incorporated into solid oral dosage forms.
Ascendia specializes in the application of nano-technology approaches to develop novel pharmaceutical products. Our suite of state-of-the-art technologies for formulating poorly water soluble compounds provides sophisticated formulation options for our clients. Contact us today for more information on how our formulations can enable your pharmaceutical product.
CASE STUDY – ASD-002 (clopidogrel for injection)
Ascendia has developed a novel nano-emulsion formulation of clopidogrel, a leading anti-thrombotic medicine that is used to treat Acute Coronary Syndrome. Unfortunately, clopidogrel is only available commercially as a solid oral dose product, and administering it in an acute, emergency situation can be difficult. Also, when delivered orally there is a significant delay in the time required for the medicine to become effective.
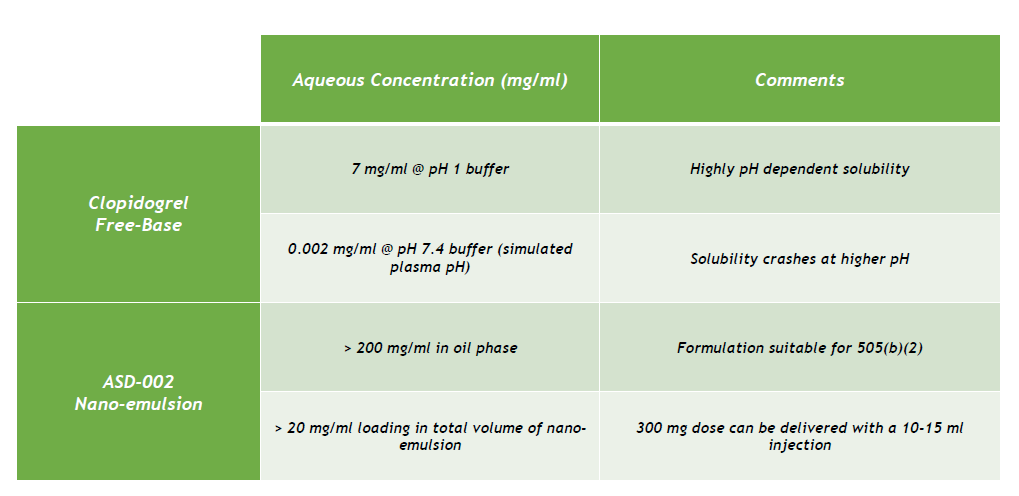
Ascendia has formulated the free-base form of clopidrogrel into a stable nano-emulsion that is suitable for injectable delivery. Since the formulation contains no solvent, the risk of injection site pain is greatly reduced. And, even though the free-base is poorly soluble at plasma pH, when contained in the oil phase of the nano-emulsion, the clopidogrel drug substance becomes much more soluble as shown in the table below.
Another challenging aspect of this development program is the demonstration of chemical and physical stability of the formulation. Clopidogrel has several chemical degradation pathways, including oxidation, hydrolysis, and chiral conversion. Ascendia has demonstrated physical stability of the formulation by showing minimal change in droplet particle size following either autoclaving the formulation, or a freeze-thaw cycle for the formulation – in either case the particle size remains a consistent 200 nm. Chiral conversion to the r-enantiomer is the predominant chemical impurity. Ascendia has shown in accelerated stability studies that chiral conversion is kept within USP limits for sufficient time to provide a commercially acceptable product shelf-life.
Want to learn more about
Ascendia's expertise?
Get up-to-date information
