New Approach to Formulation Development Can Improve Pharma Company’s Pipeline
Drug development teams at pharma companies have to change their approach to formulation development. The reason? The dramatic increase in complex molecules in the product pipeline. This altered methodology must also take into account time and cost factors, as time-to-market and budget pressures are greater than ever. Selecting the proper contract development and manufacturing organization (CDMO) can go a long way in meeting those criteria.
The importance of formulation during drug development was highlighted in a study by Rentschler Biopharma SE and LEUKOCARE AG. Among the study results:
60% of survey responders said formulation issues caused significant drug project delays and project failures
More than half of the participants stated they had clinical development delays of more than a year and 10% experienced a complete failure – all because of formulation problems
Due to the study findings, the conclusions included that many drug development teams underestimate the importance of early-stage formulations, as well as a prediction that the investment on formulation per project would double (on average) over the subsequent five years.
Advanced Technologies Improve Drug Development
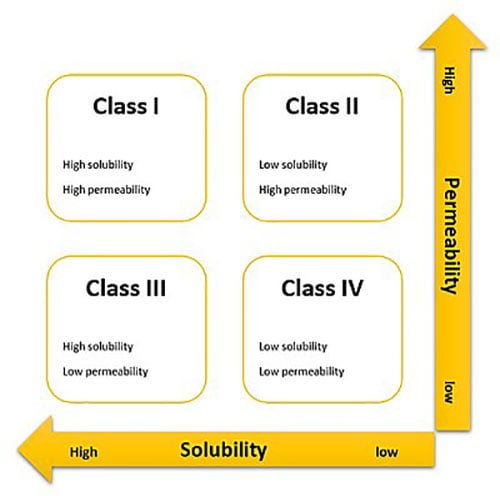
To overcome these obstacles, pharma companies are now more receptive to utilizing complex technologies. Ascendia has developed proprietary nano-technologies that have a proven track record in meeting the needs of drug development teams. These technologies have been especially effective in applications using challenging compounds, such as those classified as Biopharmaceutics Classification System (BCS) II – high permeability, low solubility – and IV – low permeability and low solubility (figure 1).
One consistent trend is that small molecules still comprise the lion’s share of drug development. The same can be said of orals and injectables in pipelines. Solubility and bioavailability difficulties are rampant in these applications, so conventional formulation approaches are not as effective as required by scientists heading drug development. They are looking for custom solutions that can best satisfy the specific molecular and route-of-administration demands of their projects.
During the early phase of a project, the probability of success to test in first-in-human or first-in-patient is the most important consideration for evaluating formulation strategy. Once the project successfully transitions to market formulation development – i.e. Phase IIb/III – other factors enter the equation. These include market acceptability or commercial aspect, scalability, intellectual property protection, and cost.
Follow the Science
A successful development program incorporates a “follow the science” philosophy when developing a formulation strategy. This includes a rational design of tox and human formulations for the precious ingredients, so a successful proof-of-concept in humans can be attained. The keys to unlock these design goals are pre-formulation of the drug candidate, biopharmaceutical evaluation, analytical method development, formulation development, and cGMP manufacturing. For clinical trials, it may also prove to be prudent to align with the commercial team’s product profile.
As mentioned at the outset of this post, time and cost are major issues that drug development teams must put front-and-center when developing formulations. Pharma companies should ask potential CDMO partners to provide proof-of-concept for a compound. This should stretch from preclinical to in-human trials as quickly as possible and on a reasonable budget. As a result, it is essential to develop highly efficient formulation strategies. In some cases, for very challenging compounds, choosing between developing the best formulation and being able to meet the budget and time demands can become extremely difficult.

Despite all these warning signs, some pharma companies don’t always establish the proper timelines for drug development. Nor do they put enough resources to establishing and following the proper formulation strategy for challenging compounds. For these reasons, selecting a more dynamic CDMO that can quickly adapt to and manage the changing scope of work proves advantageous.
Testing Compound Safety and Efficacy
The main goal in early-phase development is to test the compound’s safety and efficacy for the intended therapeutic indications in animals and humans. Significant effort must be made to improve the pharmacokinetic properties of compounds and ensure bioavailability in animal models and human Phase I and II studies. A desired compound’s systemic exposure in testing subjects is a prerequisite to meet the goals of early development. Otherwise, the project may be at a higher risk of costly failure in first-in-human and at later stages of development.
To prevent this worst-case scenario, technologies that enhance the solubility and permeability of drug candidates need to be used. They are vital in the enabling of formulation design.
Optimization of solubility and bioavailability is achievable via suitable delivery systems. The optimal system for an application is selected after screening technology platforms that address different compound challenges in hydrophilicity, lipophilicity, permeability, and melting point. Often, Ascendia will simultaneously utilize all its technologies – EmulSol, AmorSol, and NanoSol – to develop the most effective formulation. Implementing the technologies in parallel can save significant time and money. Another specific benefit of Ascendia is that its nano-technologies can accommodate any drug delivery dosage form that commercial and/or portfolio teams desire.
Selecting Best CDMO for a Project
Outsourcing partners should play a crucial and integral role in a formulation strategy. A partner can provide expertise in chemistry, manufacturing, and controls (CMC), technology, and regulatory affairs. It also can act as a traditional pharmaceutical R&D department for a pharma company. The key is to find a CDMO with the experience and depth to provide these services in a manner that will push drugs through a pipeline most efficiently.
Do you have a poorly soluble drug in your pipeline? Contact us to discuss how we can meet your formulation challenges.
